Lowest temperature recorded on Earth
The lowest temperature ever recorded at the surface of the Earth was −89.2 °C (−128.6 °F; 184.0 K) at the Soviet Vostok Station in Antarctica July 21, 1983.[1] Lower temperatures have been achieved in the laboratory, including a record low temperature of 100 pK, or 1.0 × 10-10 K in 1999.[2]
Contents |
Early cooling
In 1904 Dutch scientist Heike Kamerlingh Onnes created a special lab in Leiden with the aim of producing liquid helium. In 1908 he managed to lower the temperature to less than four degrees above absolute zero, to less than −269 °C (3 Kelvin). Only in this exceptionally cold state will helium liquefy, the boiling point of helium being at −268.94 °C. Kamerlingh Onnes received a Nobel Prize for his achievement.
Onnes method relied upon depressurising the subject gases, causing them to cool. This follows from the first law of thermodynamics;
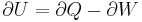
where U = internal energy, Q = heat added to the system, W = work done by the system.
Consider a gas in a box of set volume. If the pressure in the box is higher than atmospheric pressure, then upon opening the box our gas will do work on the surrounding atmosphere to expand. As this expansion is adiabatic and the gas has done work
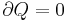
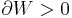
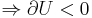
Now as the internal energy has decreased so has the temperature.
Modern cooling
As of November 2000, nuclear spin temperatures below 100 pK were reported for an experiment at the Aalto University's Low Temperature Lab. However, this was the temperature of one particular type of motion—a quantum property called nuclear spin—not the overall average thermodynamic temperature for all possible degrees of freedom.[3] At such low temperatures, the concept of "temperature" becomes multifaceted since molecular motion cannot be assumed to average out across degrees of freedom.
The current apparatus for achieving low temperatures has two stages. The first utilizes a helium dilution refrigerator to get to temperatures of millikelvins, then the next stage uses adiabatic nuclear demagnetisation to reach picokelvins.
References
- ^ http://ecolocalizer.com/2008/12/14/the-coldest-inhabited-places-on-earth/
- ^ "World record in low temperatures". http://ltl.tkk.fi/wiki/LTL/World_record_in_low_temperatures. Retrieved 2009-05-05.
- ^ The experimental methods and results are presented in detail in T.A. Knuuttila’s Ph.D. thesis which can be accessed from this site. Also the university’s press release on its achievement is here